
Publisher:
Bonnie King
CONTACT:
Newsroom@Salem-news.com
Advertising:
Adsales@Salem-news.com

~Truth~
~Justice~
~Peace~
TJP
Nov-13-2011 02:00

 TweetFollow @OregonNews
TweetFollow @OregonNews
Protecting Americans, FDA Style
Marianne Skolek Salem-News.com"He who passively accepts evil is as much involved in it as he who helps to perpetrate it. He who accepts evil without protesting against it is really cooperating with it." Martin Luther King, Jr.
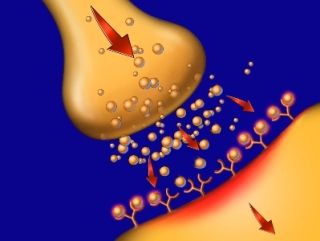 Opioids stimulate the release of "euphoric" chemicals in the brain. Over time, more drug is required to produce the same release, leading to opioid abuse. © 2009 Nucleus Medical Art, Inc. |
(MYRTLE BEACH, S.C.) - Is the FDA protecting the American consumer from a "major public health crisis" with serious harm from opioids -- or are they allowing the pharmaceutical companies such as Purdue Pharma, maker of OxyContin -- to continue the addiction, misuse, abuse, overdose and death throughout the US and Canada?
The FDA is not the watchdog protecting the American consumer -- the pharmaceutical criminals are the pit bulls perpetrating this crisis.
From the FDA website:
Opioid Drugs and Risk Evaluation and Mitigation Strategies (REMS)
Opioids are at the center of a major public health crisis of addiction, misuse, abuse, overdose and death. FDA is taking action to protect patients from serious harm due to these drugs. This action represents a careful balance between continued access to these necessary medications and stronger measures to reduce their risks.
Update on Implementation of Opioids REMS
After notifying the sponsors of long-acting and extended-release (LA/ER) opioid drugs that they were required to submit a risk evaluation and mitigation strategy (REMS), FDA has been working with the sponsors that market these products on the required REMS. The central component of the Opioid REMS is an education program for prescribers (e.g., physicians, nurse practitioners, physician assistants) so that LA/ER opioid drugs can be prescribed and used safely. FDA expects the prescriber training to be conducted by accredited, independent continuing education (CE) providers, without cost to the healthcare professionals, under unrestricted grants to accredited CE providers funded by the sponsors.
On November 4, 2011, FDA announced the availability for public comment of a draft "Blueprint." The Blueprint, developed by FDA with advice from other Federal agencies, is a basic outline and the core messages that FDA believes should be conveyed to prescribers in a basic two to three hour educational module. After it is completed and approved as part of the REMS, the Blueprint will be posted on the FDA Web site for use by CE providers in developing CE courses.
For further information, please contact: OpioidREMS@fda.hhs.gov.
If the FDA is "working with the sponsors that market these products on the required REMS" -- who is working with the victims of the lies told about the addictive dangers of OxyContin perpetrated by Purdue Pharma? If this wasn't such a tragedy in human life because of the criminal marketing of OxyContin, it would almost be satirical -- the FDA asking Purdue Pharma to present a Risk Evaluation and Mitigation Evaluation on any drug after their criminal conviction is like asking Charles Manson to write a paper on protecting the American people against home invasion.
Hello,I'm trying to find any research, investigation, or survey completed on non-cancer pain patients use of opioids before and after the Opioid Risk Evaluation and Mitigation Strategy (REMS) was added. Please let me know of these studies and where I can find the methodology and results.
Thank you,
Deb
Deborah J. Miller, Ph.D., M.P.H., R.N.
Cancer Liaison Program
FDA/OC/Office of Special Health Issues
10903 New Hampshire Ave.
Bldg. 32, Room 5332
Silver Spring, MD 20993-0002
General number: 301-796-8460
Phone: 301-796-8472
Fax: 301-847-8623
I replied tongue in cheek with the following email to "Deb" --
Hi Deb -- my suggestion would be that you check with Curtis Wright, MD -- he was a previous FDA employee responsible for the review and approval of Purdue Pharma's drug, OxyContin back in the 1990's and I'm confident he has the survey and investigation data you are looking for. Unfortunately, he is no longer with the FDA -- after OxyContin was approved by the FDA, he left your agency to work for Purdue Pharma -- an FDA conflict of interest. I'm sure he has information that would prove very helpful to the FDA before REMS was initiated and given to pharma for input. Marianne
The FDA never disappointing me -- Deb replied to my factious email as follows:Thanks so much Marianne!
I'm sure Deb Miller has no idea who Curtis Wright, MD is because it never concerned the FDA that Dr. Wright approved a very dangerous opioid and violated FDA standards and went to work for the pharmaceutical company after he reviewed and approved the drug for American consumers -- the drug being OxyContin.This week the maker of OxyContin's VP for State Government and Public Affairs - Alan Must was quoted as saying -- "The idea that our business is based on getting patients addicted and dependent is absurd -- it's not unusual for patients to become physically dependent. In the company's view (Purdue Pharma), Americans have long suffered from an epidemic of pain, and Purdue provides profound relief. At the end of the day, I am very proud to work for this company and proud of the things we have done." Further, Mr. Must stated "For those individuals who think we're evil, I don't think there's anything we can do that is going to change their opinion."
Yes Mr. Must you have to be proud of the billions of dollars your company reaped by misleading physicians and patients about the addictive qualities of OxyContin -- for which your company and its executives were criminally convicted. And no Mr. Must I and tens of thousands of people whose lives were ruined because of Purdue Pharma's not having a conscience in their marketing of OxyContin, do not think you are evil -- we know for a fact you are evil.
If the FDA continues to buy the marketing strategy that Purdue Pharma is spewing in the "epidemic of pain" in this country, their REMS should be approved quickly. Guess Purdue Pharma can only hope that Deb Miller reviews it -- since it is obvious she doesn't know how much evil truly penetrates the FDA walls.
LP - When we give each other love, faith, encouragement, laughter and peace, we are blessed with the stars and the moon every minute of every day.
_________________________________Salem-News.com Reporter Marianne Skolek, is an Activist for Victims of OxyContin and Purdue Pharma throughout the United States and Canada. In July 2007, she testified against Purdue Pharma in Federal Court in Virginia at the sentencing of their three CEO's - Michael Friedman, Howard Udell and Paul Goldenheim - who pleaded guilty to charges of marketing OxyContin as less likely to be addictive or abused to physicians and patients. She also testified against Purdue Pharma at a Judiciary Hearing of the U.S. Senate in July 2007. Marianne works with government agencies and private attorneys in having a voice for her daughter Jill, who died in 2002 after being prescribed OxyContin, as well as the voice for scores of victims of OxyContin. She has been involved in her work for the past 8-1/2 years and is currently working on a book that exposes Purdue Pharma for their continued criminal marketing of OxyContin.
Marianne is a nurse having graduated in 1991 as president of her graduating class. She also has a Paralegal certification. Marianne served on a Community Service Board for the Courier News, a Gannet newspaper in NJ writing articles predominantly regarding AIDS patients and their emotional issues. She was awarded a Community Service Award in 1993 by the Hunterdon County, NJ HIV/AIDS Task Force in recognition of and appreciation for the donated time, energy and love in facilitating a Support Group for persons with HIV/AIDS.
Marianne Skolek
National Activist for Victims of OxyContin and
Purdue Pharma - a criminally convicted pharmaceutical company
Staff Writer, Salem-News.comhttp://www.purduepharma.com/
pressroom/app/news_announc/ USGovt_reponse_Main.pdf http://judiciary.senate.gov/
hearings/testimony.cfm?id= 2905&wit_id=6612
Salem-News.com:
Quick Links
DINING
Willamette UniversityGoudy Commons Cafe
Dine on the Queen
Willamette Queen Sternwheeler
MUST SEE SALEM
Oregon Capitol ToursCapitol History Gateway
Willamette River Ride
Willamette Queen Sternwheeler
Historic Home Tours:
Deepwood Museum
The Bush House
Gaiety Hollow Garden
AUCTIONS - APPRAISALS
Auction Masters & AppraisalsCONSTRUCTION SERVICES
Roofing and ContractingSheridan, Ore.
ONLINE SHOPPING
Special Occasion DressesAdvertise with Salem-News
Contact:AdSales@Salem-News.com




Terms of Service | Privacy Policy
All comments and messages are approved by people and self promotional links or unacceptable comments are denied.
[Return to Top]
©2026 Salem-News.com. All opinions expressed in this article are those of the author and do not necessarily reflect those of Salem-News.com.